Talking about transplants
Transplants: How do they work?
in very bald men there is still a ring of hair extending around the sides and back of the head.
a hair transplant surgery follicles are obtained from donor areas at sides and back of the head and are later reinserted into the bald or thinning areas of the scalp.
what a hair transplant cannot avoid (and this is an important factor to bear in mind when designing the pattern for newly transplanted hair) is that hair continues to be lost in areas susceptible to hair loss.
and to avoid as much as possible the development of the alopecia, it’s advisable to continue using medicinal anti-DHT measures (finasteride, minoxidil).
it is almost impossible to have the same density as was there before the onset of hair loss, when we were adolescents,
Who are the most suitable candidates?
A hair transplant does not cure alopecia; it only attempts to repair those areas which have been irreversibly affected by the androgens. Therefore, surgery is not preventative but reparative.
Younger patients (less than 30 years) are generally not suitable candidates as their hair loss has not yet gone to completion, meaning that it has NOT YET STABALISED.
In men the areas with alopecia are more local
In men the donor area tends to be more extensive,
How to get the best results
Lamentably the history of transplants began with the old punch techniques (or doll´s hair grafts) which in spite of being technically inacceptable due to the unnatural results, they persisted for long enough
For years this was done using the classic system whereby the surgeon placed the implants one by one into the incisions previously made by a small scalpel. The implants placed in this way had a tendency to come out; therefore it was habitual that they were placed between 4 and 5 mm away from each other.
We believe that currently the best method of placing follicular units in the receptor area is by using the Implanter. With it, the hair enters the skin protected by the metallic sheath of the channeled needle (which can measure 0.7, 0.8, 1mm) and is deposited at the correct depth and angle in a natural fashion,
No, definitely not.
How many hairs do I need?
For example, a person who is extremely bald (Norwood 6 or 7) and with fine hair will never hope to have a transplant with any level of density.
Working with realistic expectations, the total number of implants which are required for each patient will depend on the contrast in colour between the hair and the scalp, the density of the donor area, the laxity of the scalp,
A man who is used to his baldness will be easier to keep happy and will accept a less than complete result as opposed to a younger man who is beginning to lose his hair
The goal of the hair transplant professional is to help the patient understand how close he can get to resolving his needs and personal expectations,


Choosing the right surgeon
Choosing the right surgeon
Many doctors have attempted to access the lucrative market of the hair transplant industry due to its rapid growth in recent years. Unbelievably, a paediatrician can become a hair transplant surgeon by completing a one weekend long course without the need of any kind of certification. Before making a decision to submit to a procedure be sure that it is the specialist surgeon who answers your questions and not a representative. But there are also many qualified surgeons who are recognized internationally. Our advice at injertocapilar.com is that once you decide to undergo hair transplant surgery, then investigate and study in depth the available techniques, different clinics and surgeons.
It is important to speak with or see the results of a patient who has had a transplant at the same clinic.
Our medical group injertocapilar.com, on deciding to operate only by the FUE technique, strives to achieve results by investigation, quality and adopting the most advanced techniques.
Talking about transplants
The development of hair transplant surgery
The hair autotransplant (initially skin with hair) was performed with varying degrees of success in the first years of the 19th century.
The first person to demonstrate the possibility of transplanting hairy areas in animals was Dr. Baromio, around 1804, but the first to treat hair loss by hair autotransplant was Dr. Unger in 1822. Dieffenbach published his doctoral thesis in 1822 also and in it described autotransplants of hair in animals.
The modern development of hair transplants did not occur until the last century. In 1939 a Japanese dermatologist called Shoji Okuda was the first to describe the punch technique by using a circular scalpel for grafts in patients with bad burn injuries.
Dr. Okuda transplanted grafts of skin with hair and introduced them into small apertures previously made in damaged parts of the scalp. He was able to see how the implants continued to grow in their new locations.
In 1943, another Japanese dermatologist,
Dr. Tamura, used micro-implants of 1 – 3 hairs to restore female pubic hair.
In 1943, another Japanese dermatologist, Dr. Tamura, used micro-implants of 1 – 3 hairs to restore female pubic hair. These small micro implants were obtained surgically by elliptical incisions made in the donor zone. As we can see he used a technique very similar to the one which was in use up until very recently. In spite of the fact that the work of Okuda and Tamura was published in Japanese medical journals, their procedures remained anonymous for some time due to the Second World War.
The hair transplant was re-discovered by Dr. Norman Orentriech in New York City in 1952, when he performed the first hair transplant on a male patient with androgenic alopecia. In 1959, Dr. Orentreich published his work after many years of rejection by an incredulous medical community. With him began the modern era of hair transplants. Unfortunately, his work was more orientated towards the punch technique by Okuda rather than the micro-implants used by Tamura, and so in the 1960´s hair restoration surgery began to progress, although in a somewhat incorrect direction
Factors influencing graft survival
Factors influencing graft survival
The progress made in the field of hair restoration surgery over the past 15 years has been remarkable.
Results are very natural looking and our understanding of full and receding hairlines has vastly improved. While outcomes are generally very good, with past reports of over 100% growth from grafts, experienced surgeons are still nagged by the inconsistencies of graft survival. Occasionally, grafts in an apparently excellent candidate will grow far less than 100% and the surgeon usually has no sound explanation. Many feel the answer lies within the basic fundamentals of hair restoration, but others believe that there are as of yet undiscovered factors which need to be disclosed.
X- and H-Factors
In the early 1980s, Norwood and Shiell proposed the term X-factor to describe unexplained poor survival of grafts beyond the control of the physician. They felt there was a little influence of X-factor in every case, but in 1-3% of patients it was significant. Norwood speculated that an autoimmune reaction might be involved. In 1994, Greco proposed the term H-factor to describe human errors leading to poor growth. He divided these into direct factors (manipulation, trauma) and indirect factors (drying, heat, staff fatigue).
What Affects Graft Survival?
- The best answer is: “nearly everything.” The following are some of the primary factors to consider in graft survival:
- Selection of patients whose donor hair is of sufficient quality and vigour to survive transplantation and future loss to baldness
- Selection of patients with a recipient area of sufficient health to support the grafts
- Avoidance of direct and indirect physical trauma to the grafts on the day of surgery
- Graft size and method of preparation
- Selection of the best storage solution (including additives) and the decision as to whether or not chill that solution
- Creation of recipient sites so that instrument size, density of sites, and depth of sites do not damage the recipient bed to the point that they impede survival of the grafts
- Finding the best plan of post-op care
While we are far from having the answers we seek, there are some very helpful studies and case reports to help guide us. The following is a list of categories believed to be important to survival along with pertinent reports from the literature. The holding solutions in these studies were chilled unbuffered normal saline (UNS) unless otherwise noted.
Hydration
If there is one universally accepted factor in graft survival, it is hydration. In 2000, Gandelman, et al. published an article in Dermatologic Surgery studying 12 patients whose grafts were subjected to dehydration and trauma. Grafts were left on a surgical glove for 3 minutes and then examined under the light microscope (LM) followed by scanning and transmission electron microscopic (EM) analysis, if indicated. Major dam- age was observed by all modalities after dehydration—and planted dried grafts were found not to grow. This report was followed by a study by Beehner (Forum, 2007) in which 60 1-hair grafts and 60 2-air grafts were allowed to dry on a wet Telfa pad for 16 minutes before placing. The grafts were getting stiff but were not
brittle. Survival for 1-hair FUs was 60% and for 2-hair FUs was 82%, suggesting larger grafts give some protection against dehydration. Wetting the dried grafts before placing did not help.
In a busy transplant setting, it is easy to lose a few grafts in each case from drying. Drying at the cutting stations, drying on the gloves, and undetected “popped” grafts continue to create a slight attrition from dehydration. The cure is persistent vigilance throughout the procedure.
Physical Trauma
The second part of Gandelman’s 2000 study showed no visible damage to grafts on light microscopy following trauma (bending, crushing, and stretching with forceps) and therefore EM was not performed. They admitted that LM could not necessarily rule out biological effects.
Beehner found that soft crushing of the bulbs with a needle driver (rubber sleeves over the jaws) resulted in 64% survival versus a hard crush (35%). Interestingly, hard crushing of the bulge area resulted in a 0% survival for room temperature grafts versus 36% for chilled grafts, indicating that chilling provides a slight protection against physical damage.
Beehner and Frechet (2006 Annual Scientific Meeting of the ISHRS) performed a transection study on slit minigrafts (SMG´s) in which intact SMG´s were compared with SMG´s that were transected at some point along the follicle. In Beehner’s grafts, intact SMG´s had a survival of 86% at 6 months, but dropped to 65% at 12 months; while transected minigrafts had a survival of only 49% at 6 months, dropping to 45% at 12 months. Frechet’s transected SMG´s had a survival in the range of 35%.
These studies give evidence that trauma, including transection, results in a seriously reduced survival rate.
Time Out of Body
In one of the earliest and most quoted studies on FUs, Limmer (1992) recorded the following survival rates at different times out of the body. Using at least 200 FU grafts for each time frame, the survival was: 2 hours, 95%; 4 hours, 90%; 6 hours, 86%; 8 hours, 88%; 24 hours, 79%; 48 hours, 54%. A 1% loss per hour is a rough guide according to Dr. Limmer.
- 2 hs ……… 95%
- 4 hs ……… 90%
- 6 hs ……… 86%
- 8 hs ……… 88%
- 24 hs ………79%
- 48 hs ………54%
While it might seem that time out of the body is a predictable critical factor, Unger’s study on 4mm grafts planted within 2 minutes of removal had no increased survival over those planted after an hour and no improvement over the survival of Limmer’s FU grafts planted after 8 hours. Measuring survival at 4 months, 184 of 218 hairs (84%) reinserted at 2 minutes
survived compared to 212 of 218 (97%) reinserted at 60 minutes (Walter Unger, presenting to AAD meeting, Dallas, Texas, 1977). Perhaps measuring at 8 months would have revealed a higher survival rate.
In an attempt to find a method for delayed graft re-implantation, Kurata, et al. compared organ culture survival (as measured by hair shaft elongation) for follicles stored for various periods of time at 4°C in Hanks solution, Dulbecco’s modified Eagle’s
medium (DMEM), RPMI, and saline before culture with DMEM in a CO2 chamber. The pH buffers were not identified. After 24,36, and 48 hours storage, survival in saline was significantly lower than the other solutions; however, none of the grafts grew inorgan culture after 48 hours of cold storage in any of the solutions. Ten grafts were preserved for 7days in DMEM at 4°C then planted under the panniculus carnosus in athymic mice. At 5 months, 6 grafts were still growing. It is clear that long-term storage of grafts would be a significant advancement but is still a work-in progress.
Chilling versus Non-chilling
Using unbuffered normal saline, Raposio, et al. reported an 87% survival of chilled (1°C) versus 88% room temperature (RT)
(26°C) storage of grafts for 5 hours followed by organ culture for 10 days in Williams E media. No survival was defined as loss of normal follicular architecture. The hair shaft elongation rate between the two groups was also similar.
Jiange, et al. (2005) compared chilled storage in Ringer’s solution for 1–7 days at 0°C versus 4°C followed by (1) outer root sheath culture and (2) implantation under the panniculus carnosus of athymic mice. Survival following storage at 0°C was modestly better than at 4°C for all time periods of storage for both ORS cultivation and implant survival, with both categories showing no significant growth after cold storage for 7 days. Qian, et al. reported on human hair follicles implanted into athymic mice after several periods of storage at 0°C in Ringer’s solution compared to 0°C in DMEM culture media. Growth after 24 hours of storage followed by implantation into athymic mice for 5 months was 84% for Ringer’s versus 72% for DMEM. Results were also better with Ringer’s at 48 and 72 hours, but with considerably reduced survival. No regrowth was seen after being held in either solution for 7 days. The ability to culture outer root sheath cells after 24 hours of graft storage was also better with Ringer’s (95%) versus DMEM (86%).
The value of chilling is well established in general organ transplantation. Kidneys, for example, show up to a tenfold increase in survival time in chilled storage compared to room temperature storage. Hair follicles do not appear to be as sensitive to RT, but studies indicate that there is an increasing loss sometime after 6 hours. However, studies have not been continued long enough to know at what time period the break point occurs; therefore, more research is needed to determine the maximum room temperature storage time for hair follicles.
Holding (Storage) Solutions
Beginning in the late 1950s, hair grafts have predominantly been stored in unbuffered normal saline (UNS). Some of the best results reported in our field have been with the use of this solution. But is it the best solution or are grafts just pretty resilient? When compared to other storage solutions, saline has generally shown decreased survival. There have been quite a few articles written on the subject recently, but a few brief comments will be made here.
PH: Being unbuffered, UNS has a variable pH, usually in the range of 5.0. Normal human serum has a pH of 7.4. Increasing acidity has a known negative effect on tissue survival. The effect of using UNS on follicular tissue pH is not known at this time.
Researchers will generally buffer normal saline with phosphate (PBS) before conducting tissue studies. Plasma-Lyte A has a pH of7.4, using an acetate buffer. DMEM most commonly contains a natural bicarbonate buffer and is designed to be used at 37 degrees in vitro in controlled chambers with 5-10% CO2. In open air, DMEM can become alkaline and may not be healthy for hair grafts. DMEM used in hair studies normally contains the more expensive HEPES buffer, which works well in open air
situations. Advanced intracellular balanced solutions most commonly use HEPES, particularly in those meant to be chilled as it adapts to temperature changes. It should be noted that DMEM is not specifically approved as a transplant storage media
(personal correspondence with Sigma-Aldrich Co.).
Osmolality and electrolyte balance: Osmolality of normal serum ranges from 280–310 mOsmol/L. UNS has an acceptable osmolality of 308. Advanced solutions use osmotic buffers because there is a higher concentration of impermeable solutes intracellularly versus extracellularly. Membrane pumps are altered during cold storage. Adding impermeable solutes, such as lactobionate and dextran, as osmotic buffers helps to maintain the proper balance, particularly in chilled solutions.
Additives to holding solutions: In 1998, Swineheart found no significant difference in graft survival comparing storage in saline solution vs. organic culture medium (RPMI) both at 9ºC, with a survival measured at 5 months from 82% vs 84% respectively.
Raposio, et al. (Derm Surg., 1998) reported that enhancing normal saline with ATP-MgCl and deferoxamine showed improved graft survival. Normal saline (control) was compared to the “enhanced” saline by storing grafts in these solutions at RT for 5 hours. Half of the grafts in the control and experimental groups were then placed in Williams E media and cultured in a controlled CO2 chamber for 10 days. The grafts in the enhanced solution had a 98% survival rate compared to 87% for the
control. The other half of the grafts was studied by hair shaft elongation, which showed no significant difference in survival.
Currently, work is ongoing with ATP, which normally has difficulty crossing the cell membrane. By using liposomes, ATP is able to easily enter the cells; but because the liposome incorporates into the cell membrane, the membrane can weaken with too high a concentration. In addition, the freeze-drying of the ATP needed for this process is very expensive. For these reasons, work is being conducted to determine the effectiveness of a safer, inexpensive preparation (lipo tripolyphosphate) topically for ATP supplementation during the post-operative period in hair transplantation.
Ischemia Reperfusion Injury and HT Grafts
During transplantation, tissues develop ischemia. In organs susceptible to IRI, upon reperfusion and exposure to oxygen, the conversion of hypoxanthine (a breakdown product of ATP) to xanthine releases free radicals and reactive oxygen species—and starts a cascade leading to cell death by apoptosis or sometimes necrosis. The free radicals released by apoptotic cell death (ACD) are particularly damaging to the double strands of DNA and the cell membrane, where they cause lipid peroxidation. This lipid peroxidation of the cell membrane releases malondialdehyde (MDA) and 4-hydroxy- alkenals (HAE), which are considered measurements of IRI. DNA breakdown during ACD can be measured by cytoplasmic histone-associated DNA fragments (HADF).
Most transplanted organs are surgically reconnected to the body’s blood supply and are exposed to a sudden dramatic rise in oxygen tension. In contrast to common organ transplants, hair grafts are perfused passively for at least 3 days before being re- vascularised, thus not receiving a sudden “blast” of oxygen. For this reason, some question exists whether IRI occurs in hair transplantation. Cooley used the MDA assay to test 150 grafts in 7 patients. The test grafts were placed into the scalp and later removed to complete the ischemia/reperfusion cycle and then tested against control grafts that were never re-implanted. The MDA assay in test grafts revealed MDA levels elevated 200–600% over controls. Krugluger, et al. demonstrated a dramatic rise in HADF after 36 hours of culture in serum containing DMEM culture media. In addition, HADF was significantly reduced by storage in media containing antioxidants. In yet another study, Krugluger reported better growth and less shedding after adding various antioxidants to holding solutions. While more studies are needed, there certainly appears to be reasonable evidence for the existence of IRI and ACD in hair grafts.
Platelet Rich Plasma
There is currently considerable interest in platelet rich plasma (PRP). PRP is rich in growth factors, among which are platelet derived growth factor (PDGF), transforming growth factor beta-1 (TGF ß-1), and vascular endothelial growth factor (VEGF). PRP has been used with benefit in both the donor strip and also grafts before placement. In 2005, Uebel presented a study in which grafts were dipped in the PRP created on the day of surgery from the patient’s blood. Grafts were dipped into the PRP for 15 minutes before implanting into the scalps of 23 patients. There was a 15% increase in graft survival in the PRP side compared to controls. PRP also looks promising in donor and recipient site healing. The negatives are that it is a little cumbersome and expensive to prepare.
Freezing for Long-term Graft Storage
In 2002, Adanali, et al. reported that grafts frozen for 2 weeks at –20°C (standard freezer) showed no damage under LM examination, suggesting that this might allow long-term graft preservation. In response, Jimenez performed a study of 150 grafts frozen for 1 hour, 5 days, and 7 days at –20°C before implantation. Survival after freezing for 1 hour was 20%; 5 days, 0%; and 7 days, 0%. This demonstrates the unreliability of LM to evaluate survival. At –20°C, ice crystals are constantly forming and reforming, killing the cells. Freezing tissue for storage requires much colder temperatures in order to create a “glass formation state” (no crystal movement), usually with liquid nitrogen. This is an involved process using cryoprotectants in which modifications for tissue type and timing of the freeze/thaw are critical
10 UF/cm2 ………. 97%
20 UF/cm2 ………. 92%
30 UF/cm2 ………. 70%
40 UF/cm2 ………. 79%
An important and often-quoted study on 2 patients by Mayer, et al. in 2000 compared 2-hair FUs planted at various densities and measured at 8 months. Results showed the following survival: 10/cm2, 97%; 20/cm2, 92%; 30/cm2, 70%; 40/cm2, 79%. All sites were made with an 18g needle, which is quite large by today’s standards. In a 2006 study, Beehner studied 2 patients using densities of 20 and 30/cm2 into 19g needle sites and 40 and 50/cm2 into 20g needle sites. Results showed the following: 20/cm2 (95% patient 1, 87% patient 2); 30/cm2 (93%, 92%); 40/cm2 (70%, 100%); 50/cm2 (67%, 94%). While the results are inconsistent, this study seemed to indicate that recipient site size is important. A recent yet unpublished study tends to verify this, showing 98–100% survival at densities of over 60 and 70 FUs/cm2 while using small recipient sites. Survival at higher densities is influenced by a variety of factors, the most important of which are the site size, tissue handling, donor hair quality, and recipient site quality. Doctors new to the field would be well served to increase density slowly.
Skinny vs. Chubby
In 1997, Seager performed a study on 88 “skinny” grafts in which trimming left the papillae with no surrounding tissue and compared them to 163 “chubby” grafts in which ample surrounding tissue was left. The survival rate was 89% and 113%,
respectively. In 1999, Beehner compared survival in 60 “skinny” and 60 “chubby” grafts, but left an equal amount of tissue surrounding the dermal papillae. Result survival rates were 101% and 133%, respectively. More recent studies have not shown
survivals much in excess of 100%, possibly due to better counting techniques. Regardless, it appears healthier for the grafts to leave a little tissue beyond the dermal sheath and papillae. Planting trauma and graft dehydration may be reduced with having just a little extra tissue.
Intact versus Non-intact Grafts
In 1999, Beehner performed a study comparing intact FU´s compared to grafts with the same number of hair follicles but containing follicles from two adjacent FUs that were subdivided. The grafts containing follicles from subdivided FUs actually had a slightly better survival rate, though not significant. From this study, it appears that it should be safe to divide FU´s, if needed.
Lateral (Coronal) vs. Parallel (Sagittal) Grafts
In 2006, Perez and Parsley performed a study using 2-hair grafts planted both laterally (l) and parallel (p) at densities of 30, 40, and 50 grafts/cm2. Results: 30/cm2, 70% (p) vs. 100% (l); 40/cm2, 86% (p) vs. 92% (l); 50/cm2, both 105%. All sites were made with a 19g needle. This small study, along with a general overview of results around the world, would tend to indicate that there may be no significant differences in survival using lateral versus parallel grafts.
30 UF/cm2………. P: 70%
L: 100%
40 UF/cm2 ………. P: 86%
L: 92%
50 UF/cm2 ………. P y L: 105%
Miscelaneas
In the July/August 2007 issue of the Forum (Vol. 17, No. 4), Rinaldi, et al. used a twice daily topical post-op solution containing adenosine sulfate 0.1%, taurine 1.0%, and ornithine chloride 1.0% (called 1-3 atodine). Adenosine sulfate up regulates vascular endothelial growth factor (VEGF) and follicular growth factor-7 (FGF 7), while taurine and ornithine stimulate outer root sheath growth. At 1 month, vessel diameter and hair shaft diameter were both larger than the placebo.
Revascularization (using reflectance confocal microscopy) of the grafts was quicker by nearly threefold, and the follicle growth was improved. Could one of the keys to improved graft survival reside with VEGF? Yano, et al. demonstrated that perifollicular angiogenesis correlated with up-regulation of VEGF mRNA expression in murine outer root sheath keratinocytes, but not in dermal papillae cells. The role of the ORS being the primary site of VEGF up-regulation was also found in a study by Krugluger, et al. Transgenic over-expression of VEGF resulted in a strongly induced perifollicular angiogenesis; resulting in increased hair growth, follicle size, and shaft diameter. Systemic neutralizing anti-VEGF
antibodies resulted in poor hair growth and reduced follicle size. Because the outer root sheath is more accessible to topical therapy than the dermal papillae, it is easy to speculate that the topical 1-3 atodine solution
mentioned in the previous paragraph might be effective.
General Impressions
We have looked at graft survival from many viewpoints, but we have not yet satisfactorily uncovered some of the factors leading to inconsistencies in growth. In this author’s opinion, part of it may lie in the recipient bed and the speed of revascularization. Grafts placed inmediately after harvesting don’t seem to grow significantly better than those placed several hours later. Rinaldi’s use of topical 1-3-atodine solution post-transplantation, the effects of PRP, the use of inhibitors of iNOS, and the work on up-regulating VEGF are all exciting. Grafts may take 3 or more days to re-vascularise. Anything to speed this process or support them in the interim logically might help. Preconditioning of grafts with growth factors and antioxidants while out of the body is also very promising. Additionally, isolated cases suggesting improved hair growth using hyperbaric oxygen (HBO) are encouraging, especially when one considers studies showing improved skin graft and flap survival with HBO. It should be pointed out that oxygen therapy is known to stimulate angiogenesis.
In conclusion, there is much to be learned about hair graft survival. Fortunately, interest in research is growing rapidly.
William M Parsley MD
Translation: Dra. Ximena Vila
Aesthetic treatment for hair
Maintaining great hair for life
Alopecia
Does hair renew itself, that is to say, is it normal that hair falls out?
The rate of growth in the scalp is approximately 0,035 mm per day or 1cm per month.
Why does baldness occur?
Front line reconstruction
Reconstructing the front hair line
The size and form of the head varies from one person to another and so too does the position of the hair line.
The most important factor of hair restoration surgery is that the restored hair appears as natural looking as possible. The basics for the artistic distribution of the front hair line are:
The density of the front line and the front third of the scalp have to be maximum.
Classifying hair loss
Classifying hair loss
Women suffering from androgen alopecia experience a pattern of hair loss which is somewhat different from that of men


After transplant
Signs and symptoms after a hair transplant
1st & 2nd day postoperative:
Receptor area: Apply saline solution every 1.5 hours. There may be a slight inflammation of the forehead or in between the eye brows due to the use of local anaesthetic. This will last a maximum of 3 or 4 days.
Donor area: Slight discomfort only when pressure is applied to the extraction zone. Feeling begins to return to the area and the “helmet” sensation produced on the day of the intervention from the anaesthetic disappears.
3rd day:
Receptor area: The inflammation of the forehead, if it has occurred, begins to disappear. There is a slight tightening or “cork” feeling (a decrease in sensibility) which may last around 10 – 15 days depending on the number of units transplanted. The patient begins to wash their head with Johnson´s Baby shampoo or FLEX by Revlon (for normal hair), depending on the doctor’s instructions. The hairs feel hard, like beard hair after 4 days. It is no longer necessary to apply the saline solution (although the patient may continue its use if so desired).
Donor area: Painless. The sensation of itch begins due to the healing of the tiny wounds and the shaving of the head. Saline solution may be applied and/or a sedative can be taken to relieve the sensation. A slight discomfort may appear at the nape of the neck due to the use of local anaesthetic in the area.
1st week:
Receptor area: Between the 7th and 10th day all of the scabs and flakes of skin will disappear leaving behind only a slight redness (which may last for around 20 days) as well as the newly transplanted hair. There may also be slight peeling of the skin during the next 30 days.
Donor area: The small wounds have completely healed. The hair has grown sufficiently such that it is difficult to notice there has been any kind of surgery.
2nd week:
Receptor area: The scalp is clean of scabs and flakes. The only evidence, if any at all, is a slight redness.Donor area: This area is now forgotten about, without any mark and well on the way to recuperation
2 to 8 weeks:
Receptor area: This is the phase in which the transplanted hairs begin to fall as they enter the telogen or rest phase (telogen effluvium) due to the shock of transplant. There may be a loss of up to 70%, higher or lower, depending on the person. When the units are implanted in between hairs, the native units may also suffer shock (shedding or shock loss) and enter a rest phase. Our occurrence of this is 5% and in such an event the native hairs are recovered in 3 -4 months. It is only a temporary complication.
Donor area: This area continues to recover. There are no signs of any surgery.
2 to 4 months:
Receptor area: During this stage the patient is waiting expectantly. In the majority of cases the transplanted hair begins to grow between 3.5 and 4.5 months. After undergoing slight shedding or shock loss the native hair also begins to grow.
Donor area: 4th month and this area is now completely healed. The zone can even be evaluated for another intervention if so required
4 to 8 months:
Receptor area: The transplanted hair begins to grow, firstly as fine slightly twisting hair which later takes form with more normal body, volume and texture. After the 3rd or 4th month vitamins are prescribed for hair and nails to guarantee that the growing hair has all that it needs.
8 months:
Receptor area: The transplanted hair continues to grow. We have now achieved approximately 80% of the definitive result
8 to 12 months:
Receptor area: The transplanted hair continues to grow in number (there is only around 10 – 15% so the change won’t be as evident as it was between months 4 and 8) and in thickness. If there was any change in texture in the new hair it tends to recover in these months. Definitive result is at one year from the date of intervention
What can we expect
What can we expect after undergoing a hair transplant surgery?
Many in the profession will say that we can return to work the next day. This is not entirely correct for all techniques, that is to say, the post-op and consequently the discomforts of a hair transplant depend on the technique used during the extraction phase. The individual extraction technique is minimally invasive and without incisions or stitches meaning that the discomfort felt in the donor zone is well within tolerable levels after 24 hours. The strip technique, however, is not a minor surgery; a flap of skin from 10 to 22 cm long is removed, so patients should be forewarned that the recovery period is longer.
Post-operative instructions
Have a light and easy-to-chew meal after the procedure.
Don’t take any alcoholic drink for 2 days after the procedure.
Don’t take any aspirin or any other medicine which contain acetylsalicylic acid.
Don’t take any vitamin supplements.
Take the medication recommended by injertocapilar.com and always take it after meals.
Relax for the rest of the day of the procedure.
Under no exception should you scratch or touch the small scabs (if present) in the donor or receptor zone. It’s very important to keep the scalp clean and free of germs from your hands or nails.
For those patients who are taking cortisone to prevent inflammation in the forehead, we recommend that you avoid salt in your meals during the 5 days after the procedure.
ONLY FOR CASES WHERE THE STRIP TECHNIQUE WAS USED:
To prevent any problems with the stitches in the donor area, do not perform any exercises or brisk movements. This includes leaning forward to tie your laces, as this movement can create tension on the stitches of the donor zone. No exercise during the first 3 weeks after the procedure.
ONLY FOR CASES WHERE THE STRIP TECHNIQUE WAS USED:
one of our doctors in injertocapilar.com will remove the stitches 10 – 12 days after the procedure.
IF YOU USE A HAIR PIECE: you should wait at least one week after the procedure before using it again.
INSTRUCTIONS FOR WASHING YOUR HAIR:
IMPORTANT: Do not use any chemical product on your hair for one month after the procedure (dye, hair-spray, gel, foam, etc…).
DAYS 1 & 2 POST-OPERATIVE:
Receptor Area: saline spray every 2 hours except when sleeping. No washing but the grafts should be free of blood.
Donor Area: wash the area ONCE A DAY, and very carefully with your fingers (not your nails) and using lukewarm water.
For the STRIP technique with stitches, use medical shampoo with Betadine. You will notice some discomfort, tension and slight numbness.
For the FUE technique without stitches, use pharmacy shampoo Penaten or baby shampoo, pH 5.5. You will notice very little discomfort, no tension or numbness.
DAY 3 TO 15:
Wash the hair twice daily, morning and night with pharmacy shampoo Penaten or baby shampoo, pH 5.5.
For the first 4 days use only the palms of your hands and fingers gently.
It’s IMPORTANT to gradually increase the pressure to the scalp as the days go by.
NEVER use your nails
If there are small scabs, leave the shampoo in for a few minutes before rinsing and these will disappear
If you use Minoxidil lotion you can continue to use it 3 or 4 days after the procedure.
If it irritates the scalp then reduce the dosage to just once a day or wait for another week before continuing treatment
DAY 15 TO 2 MONTHS:
Wash your hair with the aforementioned shampoo.
AFTER 2 MONTHS:
You can return to using you normal shampoo.
Medical treatment
External therapy: Minoxidil
it was approved for use in men in a 5% solution.
The 5% solution has not yet been approved for use in women
Minoxidil is a potent vasodilator
Treatment with minoxidil leads to a thickening of the hair is affected zones,
The application of minoxidil should continue as long as the hair loss is a problem for the patient, since the suspending treatment leads to a loss in effectiveness and a possible return to the initial state
Minoxidil, external solution of 2% and 5%, is used for the treatment of masculine alopecia in the front-parietal and parietal-occipital regions and for diffuse feminine alopecia,
The patient should use 1 millilitre of minoxidil solution two times a day.
The best way to apply it is to place 1 ml of the solution in a small syringe (the kind used for insulin) and perform three runs along the scalp from the crown to the front hairline or vice versa and then spread it around the whole area.
Minoxidil is very tolerable and the side effects are mainly dermatological in nature.
Systemic therapy: Finasteride
FINASTERIDE (PROPECIA) – Updates
Mode of action:
Dosage:
Adverse side effects:
- Reduction of the libido (1.8% in the finasteride group and 1.3% in the placebo group during the first year. In the fifth year these figures became 0.3 vs. 0 respectively).
- Erectile dysfunction (1.4% against 0.6% after a year and 0.3 vs. 0 respectively in the fifth year)
- Reduction in the volume ejaculated (1.4% against 0.9% which in the fifth year became 0% in both groups).

Very infrequently there have been published cases of gynecomoastia, testicular pain and hypersensitive reactions to a 1 mg dosis of finasteride which were reversible on suspension of drug.
When considered separately, there was no significant difference in adverse effects in the placebo group but there was when they are considered globally, 3.9% (finasteride group) versus 2.4 % (placebo group). The adverse effects simultaneously reduce during the months after in 58% of those who continue the treatment, they are completely reversible after suspension of the drug. A recent study confirmed that a 1 mg daily dosis of finasteride taken during 48 weeks does not affect spermatogenesis or production of sperm in males between the ages of 19 and 41.
Long term effects are still unknown, although there are no known systemic adverse effects. A study carried out by the American Urological Association on “Recommendations for the prophylactic use of finasteride against prostate cancer” demonstrated that finasteride in dosages of 5 and 1 mg reduce the size of the prostate, the levels of PSA (prostate specific antigen used to detect cancer in the prostate) and DHT. So while it can´t be said that finasteride 1 mg eliminates the risk of cancer, it does reduce its occurrence.
It´s not necessary for any specific laboratory test, but it is feasible to ask for a base analysis before prescribing the medication. In this way, previous changes can be detected, which if done later couldn´t be attributed to the drug.
Use in women – Teratogenicide:
Even though the FDA has not yet approved the use of finasteride in women, there are studies looking at the use of finasteride in women who suffer from androgenetic alopecia. Several studies have shown that finasteride 1 mg/day gave no results whereas the use of 2.5 to 5 mg/day showed more encouraging results. An example of this can be seen in a study performed by Lorizzo et al. in which there was an improvement in the alopecia in 23 out of 27 women in pre-menopause (associated with an ant conceptive composed of drospirenona and ethinly estradiol). Another study was presented by Trueb (5 post-menopausal women treated with 2.5 or 5 mg finasteride) which showed positive results. These are only examples but there is still a long way to go and much research to do.
Finasteride is categorized as X for pregnant women, as being an anti-androgen means that it can feminize a masculine fetus. Its use in women of fertile age is strictly forbidden unless they are using anti-conceptive measures. Women of fertile age not only shouldn´t take finasteride nor should they touch broken or squashed tablets. Nevertheless, unless there is a breakage in the women’s skin, significant percutaneous absorption is unlikely to occur. Finasteride tablets have an impermeable coating which prevents contact with the active ingredients during handling. In theory there is a risk, although highly improbable, of deformations of the sexual organs of the masculine fetus if the couple have sexual relations during the stages of pregnancy when the sexual organs of the fetus are developing (in the 8th and 15th week of gestation). That said the quantity of the drug found in semen is very small and considered to be insufficient to cause any damage to the masculine fetus. A report from a laboratory that fabricates finasteride stated that they measured the concentrations in semen of 35 males who were taking 1 mg per day of finasteride during 6 weeks. In 60% of the samples (21 of 35) they could not detect any finasteride. Therefore, when using finasteride, only a very very small part of it goes to the semen. However, even if it weren´t so low, it still wouldn´t cause any problems. The transfer of finasteride, from semen to the women through the vaginal wall was not detected in the many examinations performed to date. The risk of teratogenicide in humans has not been evaluated, only in experimental animals, in which abnormalities were caused in the urogenital system. To date there are no studies which evaluate if the oral ingestion of semen of a person taking finasteride by a pregnant woman gives rise to sufficient absorption via the intestine to cause any effect on the fetus.
Lactation: finasteride is not recommended for women during the lactation period. It is not known if finasteride is excreted in mother’s milk.
Evaluation of sexual function in patients taking Finasteride
Tosti, B.M. Piraccini, M. Soli
Department of Dermatology and Urology, University of Bologna, Italy. European Academt of Dermatology and Venereal Diseases (2001) 15, 418-421
Introduction
1 mg Finasteride 1 mg (PROPECIA) has been approved in the USA and in the majority of European countries for the treatment of androgenic alopecia in its early stages in men.
The main concern that many doctors and patients have about this drug is the possible
appearance of adverse affects that alter the sexual function, an observation that has been noted in 2% of the patients
participating in different studies performed on the drug’s effectiveness. The sexual side effects include a decrease in libido, erectile dysfunction and a reduction in the volume ejaculated.
Our practical experience, however, in a clinic specializing in hair transplants, shows that the adverse effects of a sexual nature occur in less than 0.5% of the patients that take finasteride 1 mg.
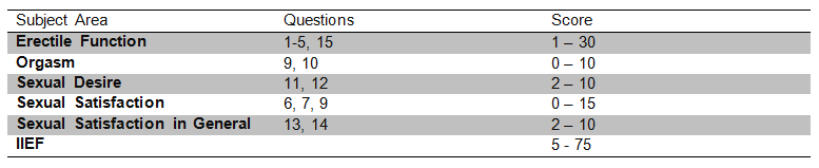
The objective of this study is to evaluate the sexual function of patients taking 1 mg finasteride compared to the control patients (without medication and of similar age) using the International Index of Erectile Function (IIEF). The IIEF is a valid means of evaluating the masculine erectile function cross-culturally as well as psychologically, which has been used to evaluate the effectiveness of sindenafil (VIAGRA) and phentolamine as oral treatments for erectile dysfunction. The IIEF is a brief questionnaire consisting of 15 questions, is reliable, performed by the patient himself, and is available in 10 languages (table 1). Five main areas are evaluated: erectile function, orgasmic function, sexual desire, satisfaction from the act of sex and satisfaction sexual in general. The sum of the points gained in each area produce a result which when added corresponds to the final score of the IIEF, the highest score being related to a better sexual function (table 2). The subject area is considered a worthy diagnostic tool to distinguish between patients with and without sexual dysfunction. A score of erectile function less than 25 indicates erectile dysfunction.
Method and materials
Our study includes 236 patients, between 18 and 47 years of age, who were monitored as patients with androgenic alopecia taking 1 mg finasteride. All of the patients were informed of the study, they willingly accepted to participate and they themselves answered the questions. At the time of completing the questionnaire, the average time that the patients were taking finasteride was 8 months (between 6 and 13 months). The control patients include 236 men of similar age who were attended in our hospitals for disorders of the nails. None of the control patients were taking any kind of medicine which could produce or interfere with their sexual function in any way. A statistical analysis model was used to compare the scores obtained in the questionnaires of the IIEFF in both groups of patients.
Table 1. Individual questions from the IIEF questionnaire.
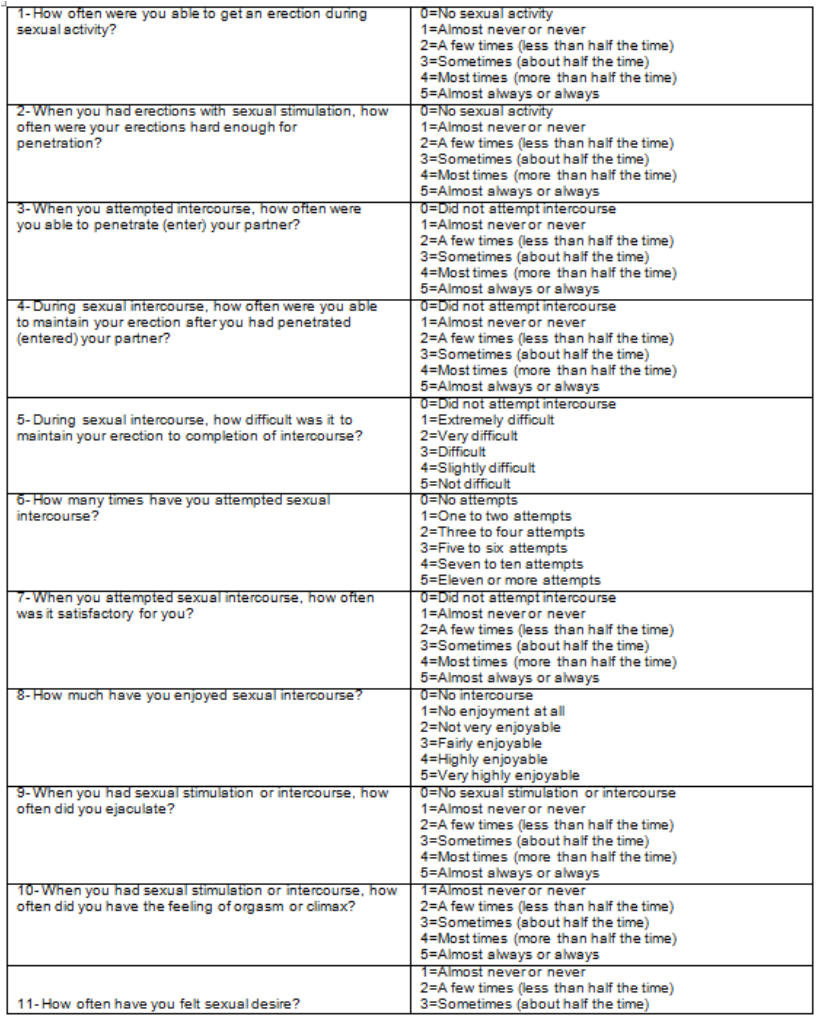
Table 2. International Index of Erectile Function
Results
The comparison between the scores obtained in the group taking finasteride and the control group did not demonstrate any statistically significant differences with respect to the general IIEF result or when taking each subject area separately (fig 1). This indicates that the sexual function as well as the erectile function of the subjects taking finasteride is not reduced with respect to the control group (patients without medication).
Figure 1. Graph which compares the erectile function (EF) and the global results of the IIEF questionnaire in patients treated with 1 mg finasteride and the control patients.


Discussion
Finasteride is an inhibitor of Type II 5-alfa-reductase, inhibiting the conversion of testosterone into dihydrotestosterone (DHT). The drug is effective in the treatment of androgenic alopecia, where follicular miniaturization occurs as a consequence of the action of the androgens (especially and mainly DHT) on the follicular units.
The administration of 1 mg finasteride (PROPECIA) significantly reduces the levels of DHT in plasma, prostate and scalp and produces a slight increase in the levels of testosterone that, however, stays within physiological limits. As it is the testosterone and not the DHT that is responsible for sexual function after puberty, then
in theory by administering Finasteride a response that leads to sexual dysfunction is not expected.
Nonetheless, the adverse effects of a sexual nature were obtained in around 2% of various clinical studies that evaluated patients taking 1 mg finasteride for androgenic alopecia. The act of informing patients about the possible interference of the drug with the sexual function can bias those results to a certain extent. The doctors who prescribe this drug rarely observe side effects of a sexual nature.
Our study was completed evaluating the sexual function of individuals taking 1 mg finasteride using an objective method, the IIEF questionnaire. In spite of the fact that the patients taking finasteride were informed of the possible adverse side effects, the results indicate that the sexual function as well as the erectile function of these patients was not reduced with respect to the control group. These results are in accordance with the experience of many dermatologists who do not observe erectile or sexual dysfunction in patients who take finasteride 1 mg.
We believe that the IIEF questionnaire should be performed routinely on patients who begin treatment with 1 mg finasteride, with the goal of being able to include a quantitative method to evaluate the sexual function during treatment. Our experience was that the questionnaire was very well accepted by the patients, who were often quite anxious about the possible side effects of a sexual nature and who were eager to have close and careful monitoring during their treatment
Folicular unit
Is all hair the same?
It is fundamental for a hair surgeon to have a complete and thorough knowledge of the anatomy and aesthetic of hair in order to be able to achieve the best results.
The form and texture of hair varies depending on its stage of evolution and position
There are several different kinds of hair:
- The so-called lanugo is fine hair, generally fair and not pigmented which covers the foetus and which usually falls out around the eight month of gestation
- Down hair or fuzzy hair is fine soft hair and almost invisible which can be observed in the forehead and scalp during pre-puberty
- Terminal hair characterizes adulthood and is normally thick, long and of varied pigmentation. There are different subcategories of terminal hair and these can be found in the scalp, eyebrows, upper lip, chin, armpit, chest and pubis
Down or fuzzy hair can become terminal hair;
for example,
fuzzy hair on the face of teenagers can change into a beard.
Terminal hairs on the scalp can also turn into down hair, as is the case in male pattern baldness and female androgenic alopecia
What is a follicular unit?
When we perform a cross section cut of a hair and study it under the microscope, we observe that the hair grows in follicular units. A follicular unit (F.U.) is composed of:
- Between one and four terminal hairs
- One soft or down hair (rarely two)
- Up to nine sebaceous glands
- The pili arrector muscles
- A perifollicular vascular plexus, and a perifollicular neural net
This suggests to a certain extent that the follicular unit constitutes a physiological entity. In a transplant, the most natural method is to insert the follicular units and therefore avoid the poor aesthetics of the old punch or mini-graft techniques, where various F.U.´s were inserted together at the same point. As well as that, and considering the aesthetics of follicular anatomy, we know that the closer we get to the frontal hair line, the less number of hairs there are in each F.U. (mono-capillary) and as we move back towards the occipital zone, the F.U.´s contain 3 and 4 hairs (for greater density).
Transplant
Is everyone a candidate for a hair transplant?
Patients should have realistic expectations and should understand that, given there is currently no method for creating hair, the current techniques aim to redistribute the existing hair in as natural a way as possible. It is for this reason that
candidates are limited by a favourable donor zone
with sufficient density relative to the area to be transplanted.
Regrettably, male pattern baldness is a progressive condition. The rate of hair that is lost may diminish after reaching 40 years of age but will never completely stop.
Therefore the pre-surgical design must ensure a result that is natural looking in the long-term.
There is greater demand in patients under 30 years of age. This kind of patient with relative air loss must understand that, due to their young age, it is impossible to determine to what extent will be their hair loss. Said patient requires a more thorough evaluation to decide between the conservative medical treatment or the alternative of grafts plus medical treatment to stop hair loss and conserve existing native hair.
How much hair can we transplant?
The scalp has a surface area of approximately 500 cm2 (50.000 mm2) and an average of 200 hairs per cm2 (80 – 100 F.U.´s). As a scalp with hair has one follicular unit per mm2 and each unit has an average of 2 hairs (for example an average of two hairs per mm2)there is a total of around 100.000 hairs. This number varies of course, from patient to patient and depending on the ethnic background.
The best donor area is where there is hair that is permanently growing and which is genetically programmed to grow throughout the patient’s life,
these are the temporal and occipital regions. At the most, we can remove 50% of the hair present in the donor areas without causing these areas to appear thin or lacking in density. In theory, in the case of moderate male hair-loss patterns (type V or VI), 12.5% of the scalp is safely available to be transplanted. This is equal to 12.500 hairs or approximately 3.000 to 4.000 F.U.´s.
The appearance of fullness has much to do with the mass of the hair. This is related to the number of hairs, the thickness of the individual hairs and locks of hair, texture, colour and whether it is straight or curly. On top of that, the contrast in colour between the scalp and the hair also significantly influences the optical illusion of fullness.
Nowadays most experts agree that the average hair density in patients, who do not suffer from hair-loss, is 200 hairs per cm2
(the range goes from 130 to 280) and that only 50% of this number is required to achieve the appearance of normal density, that is 100 hairs per cm2 ( the range goes from 65 to 140). Put another way this is an average of 45 F.U.´s per cm2.



